Meet compliance challenges with secure data preservation
Secure immediate access to your most critical digital assets and records over decades

Secure actionable data over decades
Our Active Digital Preservation™ technology eliminates the risk of long-term data loss by automatically maintaining your most critical records & assets in always readable formats
Meet compliance challenges
Demonstrate long-term ESG and quickly retrieve actionable records for compliance and regulatory audits
Protect brand value
Build value with authentic long-term assets for deeper storytelling, retro-branding and employee engagement
Drive innovation
Document and inspire new product innovation. Protect Intellectual Property (IP) and defend Trademarks
Embed in Microsoft 365
Empower users by making records preservation & retrieval a seamless part of the Microsoft 365 experience
Trusted by brands & corporations around the world
Preservica is trusted by businesses worldwide to meet the highest levels of security, reliability and performance.
AI-powered assistance across business content workflows
Integrated AI with human oversight to make it easy to get more done and improve quality of AI assistance
Categorize and describe your image collections at scale
Clear your corporate heritage backlog and save time by automatically detecting people, places, objects & more, and automating the creation of metadata.
Stay in control of how & where AI is applied across your business assets
Prevent sensitive content being exposed to AI and keep an audit of AI and human actions.
Appraise and standardize metadata across your business collections in minutes
Quickly clean-up metadata from different department contributors and resolve inconsistencies to make content easily findable.
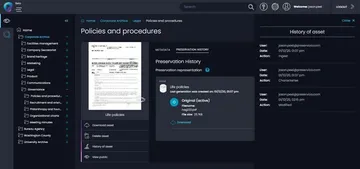
Quickly retrieve compliance and governance records when needed
- Evidence decision making, policies, reports, shareholdings and ESG
- Rapidly meet “burden-of-proof” challenges for regulatory audits
- Enable compliance @scale with seamless archiving for Microsoft 365

Reuse authentic brand assets over decades
- Create anniversary storytelling and social media campaigns
- Engage employees and share your rich history with public access
- Reuse oral histories, videos, photographs and marketing assets
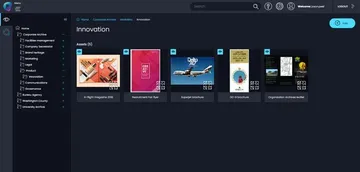
Inspire and protect new product innovation
- Document product innovation and reuse knowledge long-term
- Inspire new innovation, retro-products and protect IP
- Demonstrate "proof-of-use" for Trademark defense
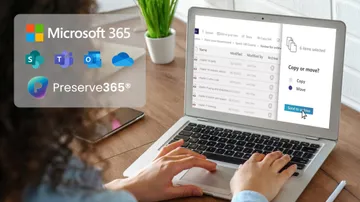
Simplify compliance and archiving with Preserve365®
- Make Active Digital Preservation part of the Microsoft 365 experience
- Simplify compliance with easy move, copy & search inside SharePoint
- No need to learn and use separate archiving software

“The corporate archive is an invaluable long-term resource for the Associated Press. With Preservica, people are astounded by the speed we can now provide information to the organization. That accessibility has proved to be indispensable and is really valued by our stakeholders.”
Valerie Komor, Director, AP Corporate Archives

“Critical digital information is being created every day, at high volume. Preservica helps us govern information over the long-term and integrates with our existing systems to give a single, cohesive view of our most important information assets.”
Tina Staples, Head of Global Archives, HSBC
A family of solutions for all your preservation & archiving needs
Start for free. Grow and expand with your needs. Make digital preservation a seamless part of your Microsoft 365 records lifecycle.
Starter
Everything you need to start preserving and sharing your digital records
Professional
Active Digital Preservation™ for larger archives with our advanced features

Enterprise
Performance, security and integration in a dedicated private cloud

Preserve365®
Make records preservation & retrieval part of the Microsoft 365 experience


Long-term security by design
Preservica is purpose built for the unique requirements of long-term data security, availability, integrity and privacy. We are ISO 27001, SOC 2 Type 2 and Cyber Essentials certified and our Enterprise edition is hosted in a secure dedicated private cloud on AWS or Microsoft Azure.

NEW! Archiving & digital preservation that's part of the Microsoft 365 experience
Learn more about Preserve365®












